FY 2012 Congressional Budget Justification
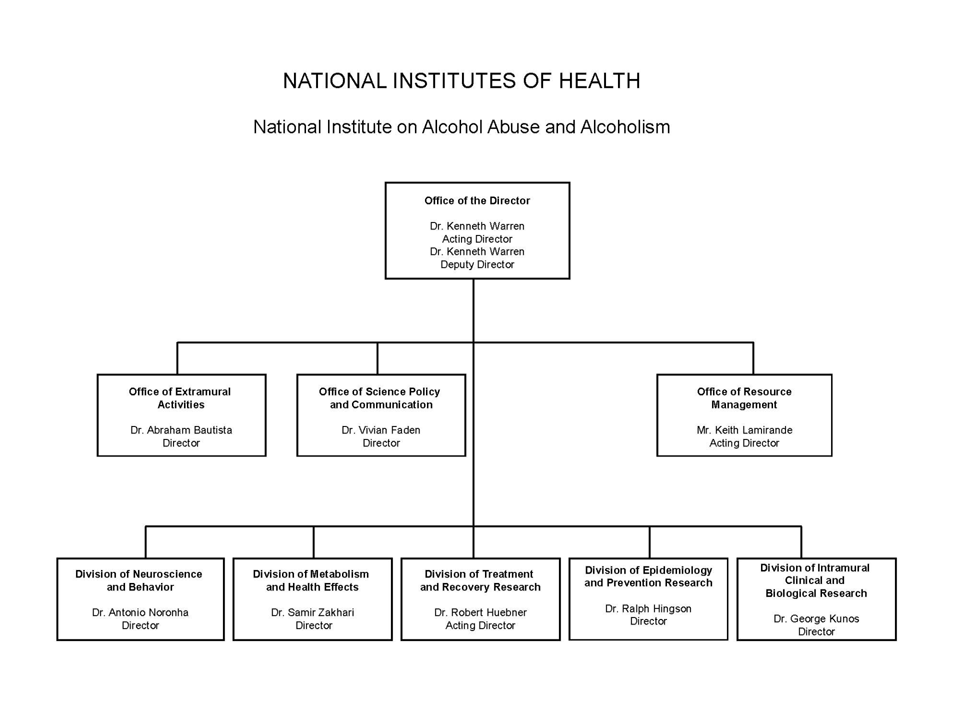
NATIONAL INSTITUTES OF HEALTH
National Institute on Alcohol Abuse and Alcoholism
For carrying out section 301 and title IV of the Public Health Services Act with respect to alcohol abuse and alcoholism, $469,197,000.
NATIONAL INSTITUTES OF HEALTH
National Institute on Alcohol Abuse and Alcoholism
Amounts Available for Obligation 1
(Dollars in Thousands)
| Source of Funding | FY 2010 Actual |
FY 2011 CR |
FY 2012 PB |
|---|---|---|---|
| 1 Excludes the following amounts for reimbursable activities carried out by this account: FY 2010: $3,551, FY 2011: $5,060, FY 2012: $5,255 |
|||
| Appropriation | 462,346 | 462,346 | 469,197 |
| Type 1 Diabetes | 0 | 0 | 0 |
| Rescission | 0 | 0 | 0 |
| Supplemental | 0 | 0 | 0 |
| Subtotal, adjusted appropriation | 462,346 | 462,346 | 469,197 |
| Real transfer under Director's one-percent transfer authority (GEI) | (704) | 0 | 0 |
| Real transfer under Secretary's one-percent transfer authority | (69) | 0 | 0 |
| Comparative Transfers to NLM for NCBI and Public Access | (191) | (393) | 0 |
| Comparative transfer under Director's one-percent transfer authority (GEI) | 704 | 0 | 0 |
| 0 | 0 | ||
| Subtotal, adjusted budget authority | 462,086 | 461,953 | 469,197 |
| Unobligated balance, start of year | 0 | 0 | 0 |
| Unobligated balance, end of year | 0 | 0 | 0 |
| Total obligations | 462,057 | 461,953 | 469,197 |
| Subtotal, adjusted budget authority | 462,086 | 461,953 | 469,197 |
| Unobligated balance lapsing | (29) | 0 | 0 |
NATIONAL INSTITUTES OF HEALTH
National Institute on Alcohol Abuse and Alcoholism
Budget Mechanism - Total 1/
(Dollars in Thousands)
|
MECHANISM |
FY 2010 Actual |
FY 2011 CR |
FY 2012 PB |
Change vs. FY 2010 |
||||
|---|---|---|---|---|---|---|---|---|
|
Total, NIAAA |
224 |
$462,086 |
224 |
$461,953 |
224 |
$469,197 |
0 |
$7,111 |
|
Research Grants |
No. |
Amount |
No. |
Amount |
No. |
Amount |
No. |
Amount |
| Research Projects |
517 |
$200,035 |
536 |
$209,296 |
471 |
$194,370 |
(46) |
($5,665) |
| Noncompeting: Administrative Supplements | 49 | $3,417 | 47 | $3,417 | 47 | 3,500 | (2) | 83 |
| Competing: Renewal | 21 | $10,077 | 17 | $8,293 | 22 | 10,750 | 1 | 673 |
| Competing: New | 168 | $57,307 | 134 | $46,608 | 172 | 60,531 | 4 | 3,224 |
| Competing: Supplements | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
Research Centers |
21 |
$28,004 |
21 |
$28,424 |
21 |
$28,705 |
0 |
$701 |
| Specialized / Comprehensive Clinical Research | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Biotechnology | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Comparative Medicine | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Research Centers in Minority Institutions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
Research Centers |
21 |
$28,004 |
21 |
$28,424 |
21 |
$28,705 |
0 |
$70 |
|
Other Research |
93 |
$14,483 |
93 |
$14,700 |
93 |
$14,847 |
0 |
$364 |
| Cancer Education | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cooperative Clinical Research | 2 | 9,173 | 1 | 6,811 | 1 | 6,880 | (1) | (2,293) |
| Biomedical Research Support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Minority Biomedical Research Support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 21 | 6,340 | 24 | 7,955 | 24 | 8,035 | 3 | 1,69 |
|
Other Research |
116 |
$29,996 |
118 |
$29,466 |
118 |
$29,762 |
2 |
($234) |
|
Total Research Grants |
867 |
$338,096 |
847 |
$333,784 |
826 |
336,018 |
(41) |
($2,078) |
|
Research Training |
FTTPs |
FTTPs |
FTTPs |
|||||
| Individual Awards | 107 | $3,816 | 107 | $3,876 | 107 | $4,000 | 0 | $184 |
| Institutional Awards | 182 | 8,026 | 182 | 8,155 | 182 | 8,415 | 0 | 389 |
|
Subtotal, Competing |
189 |
$67,384 |
151 |
$54,901 |
194 |
$71,281 |
5 |
$3,897 |
|
Subtotal, RPGs |
706 |
$270,836 |
687 |
$267,614 |
665 |
$269,151 |
(41) |
($1,685) |
|
SBIR/STTR |
24 |
$9,260 |
21 |
$8,280 |
22 |
$8,400 |
(2) |
($860) |
|
Research Project Grants |
730 |
$280,096 |
708 |
$275,894 |
687 |
$277,551 |
(43) |
($2,545) |
|
Research & Development Contracts |
70 |
$35,739 |
70 |
$37,972 |
70 |
$41,816 |
0 |
$6,077 |
| (SBIR/STTR) | 4 | $1,520 | 5 | $2,500 | 5 | $2,522 | 1 | $1,002 |
| FTEs | FTEs | FTEs | FTEs | |||||
| Intramural Research | 107 | $47,788 | 107 | $48,887 | 107 | $49,376 | 0 | $1,588 |
| Research Management and Support | 117 | 28,621 | 117 | 29,279 | 117 | 29,572 | 0 | 951 |
| Construction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Buildings and Facilities | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
Total Research Training |
289 |
$11,842 |
289 |
$12,031 |
289 |
$12,415 |
0 |
$573 |
Major Changes in the Fiscal Year 2012 Budget Request
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail and these highlights will not sum to the total change for the FY 2012 budget request for NIAAA, which is $7.111 million more than the FY 2010 Appropriation, for a total of $469.197 million.
Research Project Grants (-$2.545 million, total $277.551 million): NIAAA will support a total of 687 Research Project Grant (RPG) awards in FY 2012. Noncompeting RPGs will decrease by 46 awards and $5.7 million and competing awards will increase by 5 awards and $3.9 million. The NIH budget policy for RPGs in FY 2012 is to provide a one percent inflationary increase in noncompeting awards and a one percent increase in the average cost for competing RPGs.
Research Training (+$0.573 million, total $12.415 million): The success of biomedical research is dependent upon the robustness of NIH training programs for the next generation of scientists. NIH will provide an across-the-board increase in FY 2012 of four percent for stipends levels under the Ruth L. Kirschstein National Research Service Award training program to continue efforts to attain the stipend levels recommended by the National Academy of Sciences. This will build on the two percent increase in stipend levels for FY 2011. Stipend levels were largely flat for several years and the requested increase will help to sustain the development of a highly qualified biomedical research workforce.
Research and Development Contracts (+ $6.077 million, total $41.816 million): Funds are included in R&D contracts to reflect the NIAAA share of trans-NIH initiatives, such as the Therapies for Rare and Neglected Diseases program (TRND), the Basic Behavioral and Social Sciences Opportunity Network (OppNet), and support for a new synchrotron at the Brookhaven National Laboratory. For example, each IC that will benefit from the new synchrotron will provide funding towards the NIH total $10.0 million commitment to support this technology.
Intramural Research and Research Management and Support (+$2.539 million; total $78.948 million): Intramural Research and Research Management and Support will receive a modest increase to offset the cost of pay and other increases, including rent and utilities. For the Intramural Research Program, these additional funds are required to maintain continued support for NIAAA laboratories within the Division of Intramural Clinical and Biological Research as well as the Intramural Office of Laboratory Animal Science. For Research Management and Support, they are necessary to provide for the effective administration, planning and evaluation, public information and communications, and scientific leadership of the institute.
NATIONAL INSTITUTES OF HEALTH
National Institute on Alcohol Abuse and Alcoholism
Summary of Changes
(Dollars in Thousands)
| CHANGES | 2012 Estimate |
Change from FY 2010 |
|---|---|---|
| FTEs Budget Authority | FTEs Budget Authority | |
| Subtotal, Built-in | $498 | |
| FY 2010 Actual | $462,086 | |
| FY 2012 Estimate | 469,197 | |
|
Net change |
$7,111 | |
| A. Built-in: 1. Intramural Research: |
||
|
a. Annualization of January 2010 pay increase |
$18,764 |
$112 |
| b. January FY 2012 pay increase | 18,764 | 0 |
| c. One less day of pay (n/a for 2011) | 18,764 | (72) |
| d. Payment for centrally furnished services | 7,759 | 77 |
| e. Increased cost of laboratory supplies,materials, and other expenses | 22,853 | 223 |
| Subtotal | $340 | |
|
2. Research Management and Support: |
||
| a. Annualization of January 2010 pay increase | $17,114 | $103 |
| b. January FY 2012 pay increase | 17,114 | 0 |
| c. One less day of pay (n/a for 2011) | 17,114 | (66) |
| d. Payment for centrally furnished services | 1,292 | 13 |
| e. Increased cost of laboratory supplies,materials, and other expenses | 11,166 | 108 |
| Subtotal | $158 |
NATIONAL INSTITUTES OF HEALTH
National Institute on Alcohol Abuse and Alcoholism
Summary of Changes—continued
|
CHANGES |
2012 Estimate |
Change from FY 2010 |
||
|---|---|---|---|---|
|
No. |
Amount |
No. |
Amount | |
|
B. Program: |
||||
| a. Noncompeting | 471 | $197,870 | (46) | ($5,582) |
| b. Competing | 194 | $71,281 | 5 | $3,897 |
| c. SBIR/STTR | 22 | 8,400 | (2) | ($860) |
|
Total |
687 |
$277,551 | (43) | ($2,545) |
|
2. Research Centers |
21 |
$28,705 | 0 | $701 |
| 3. Other Research | 118 | 29,762 | 2 | (234) |
| 4. Research Training | 289 | 12,415 | 0 | 573 |
| 5. Research and development contracts | 70 | 41,816 | 0 | 6,077 |
|
Subtotal, Extramural |
$390,249 $4,572 |
|||
| FTEs | FTEs | |||
| 6. Intramural Research | 107 | $49,376 | 0 | $1,248 |
| 7. Research Management and Support | 117 | 29,572 | 0 | 793 |
|
Subtotal, program |
224 $469,197 0 $6,613 |
|||
|
Total changes |
224 $469,197 0 $7,111 |
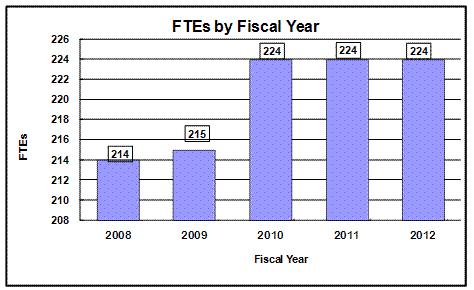
History of Budget Authority and FTEs:
Bar chart indicating FTE's for NIAAA by Fiscal Year from 2008 through 2012.
214 FTE - 2008, 215 FTE - 2009, 224 FTE – 2010, 224 FTE – 2011, 224 FTE - 2012
Bar chart indicating funding levels (Dollars in Millions) for NIAAA from 2008 through 2012.
$438.5 - 2008, $450.1 - 2009, $462.1 - 2010, $462.0 - 2011. $469.2 - 2012
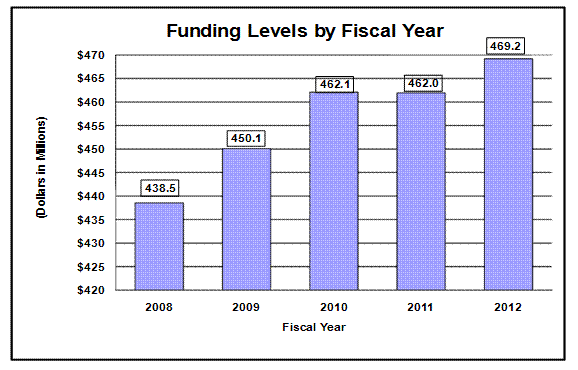
Distribution by Mechanism
Pie chart indicating funding for fiscal year 2012 by budget mechanism.
The pie chart has 7 slices. From largest to smallest the amounts are: Research Project Grants, $277.6 – 59.2%;
Intramural Research, $49.4 – 10.5%; R&D Contracts, $41.8 – 8.9 %; Other Research, $29.8 – 6.3%;
RM&S, $29.6 – 6.3%; Research Centers, $28.7 – 6.1%; Research Training, $12.4 – 2.6%
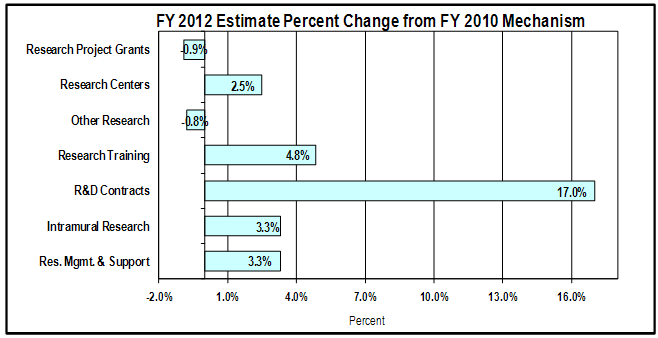
Change by Selected Mechanism:
Bar chart showing percent change by mechanism.
There are 7 bars. From top to bottom they are: Research Project Grants, 0.9% decrease;
Research Centers, 2.5% increase; Other Research, 0.8% decrease; Research Training, 4.8% increase;
R&D Contracts, 17.0% increase; Intramural Research, 3.3% increase; Resource Management and Support, 3.3% increase.
|
|
FY 2010 Actual |
FY 2011CR |
FY 2012 PB |
Change vs. FY 2010 |
||||
|---|---|---|---|---|---|---|---|---|
|
Extramural Research Detail: |
FTEs |
Amount |
FTEs |
Amount |
FTEs |
Amount |
FTEs |
Amount |
| Embryo and Fetus |
$20,266 |
$20,167 | $20,507 | $241 | ||||
| Youth/Adolescence | 60,783 | 60,485 | 61,504 | $721 | ||||
| Young Adult | 172,274 | 171,430 | 174,315 | $2,041 | ||||
| Mid-Life/Senior | ||||||||
| Adult | 132,354 | 131,705 | 133,923 | $1,569 | ||||
|
Subtotal, Extramural |
385,677 |
|
$383,787 |
|
$390,249 | $4,572 | ||
|
Intramural Research |
107 | $47,788 |
107 |
$48,887 |
107 |
$49,376 |
0 |
$1,588 |
|
Research Management & Support |
117 | $29,279 |
117 |
$29,279 |
117 |
$29,572 |
0 |
$951 |
|
TOTAL |
224 | $462,086 |
224 |
$461,953 |
224 |
$469,197 |
0 |
$7,111 |
|
1. Includes FTEs which are reimbursed from the NIH Common Fund for Medical Research. 2. Includes Real Transfers and Comparable Adjustments as detailed in the "Amounts Available for Obligation" table. |
Authorizing Legislation
NATIONAL INSTITUTES OF HEALTH
National Institute on Alcohol Abuse and Alcoholism
Authorizing Legislation
|
PHS Act/ |
U.S. Code |
2011 Amount |
FY 2010 |
2012 Amount |
FY 2012 |
|
|---|---|---|---|---|---|---|
|
Research and Investigation |
Section 301 |
42§241 |
Indefinite |
$462,086,000 |
Indefinite |
$469,197,000 |
|
National Instituse on Drug Abuse |
Section 401(a) |
42§281 |
Indefinite |
$462,086,000 |
Indefinite |
$469,197,000 |
|
Total, Budget Authority |
$462,086,000 |
$469,197,000 |
NATIONAL INSTITUTES OF HEALTH
National Institute on Alcohol Abuse and Alcoholism
Appropriations History
|
Fiscal Year |
Budget Estimate to Congress |
House Allowance |
Senate Allowance |
Appropriation |
|---|---|---|---|---|
|
2003 |
$416,773,000 |
$401,933,000 |
$418,773,000 |
$418,773,000 |
| Rescission | ($2,722,000) | |||
| 2004 |
$430,121,000 |
$430,121,000 |
431,521,000 |
$431,471,000 |
| Rescission | ||||
| 2005 |
$441,911,000 |
$441,911,000 |
$444,900,000 |
($2,802,000) |
| Rescission | $441,911,000 | |||
| 2006 |
$440,333,000 |
$440,333,000 |
$452,271,000 |
($3,634,000) |
| Rescission | $440,333,000 | |||
| 2007 |
$433,318,000 |
$433,318,000 |
$433,318,000 |
|
| Rescission | ($4,403,000) | |||
| 2008 |
$436,505,000 |
$436,505,000 |
$436,505,000 |
$435,930,000 |
| Rescission | $0 | |||
| Supplemental | $444,016,000 | |||
| 2009 |
$436,681,000 |
$451,688,000 |
$448,834,000 |
($7,757,000) |
| Rescission | $2,320,000 | |||
| 2010 |
$455,149,000 |
$466,308,000 |
$457,887,000 |
$450,230,000 |
| Rescission | $0 | |||
| 2011 |
$474,649,000 |
$473,904,000 | $462,346,000 | |
| Rescission | $0 | |||
| 2012 | $469,197,000 |
Justification of Budget Request
National Institute on Alcohol Abuse and Alcoholism
Authorizing Legislation: Section 301 and title IV of the Public Health Service Act, as amended.
Budget Authority (BA):
| FY 2010 Actual |
FY 2011 Continuing Resolution | FY 2012 Budget Request | FY 2012 +/ - FY 2010 | |
|---|---|---|---|---|
| BA | $462,086,000 | $461,953,000 | $469,197,000 | +7,111,000 |
| FTEs | 224 | 224 | 224 | + 0 |
| Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other. |
Director’s Overview
The National Institute on Alcohol Abuse and Alcoholism (NIAAA) is the lead agency for U.S. research on alcohol abuse, alcoholism, and other health and developmental effects of alcohol use. Its mission is to support research, and then translate and disseminate research findings to reduce alcohol-related problems.
Research Defines the Scope of Alcohol’s Health Effects
- Alcohol is a major cause of preventable death in the U.S. [1]
- Alcohol dependence and problem use is among the 10 leading causes of death and disability worldwide. [2]
- Alcohol problems cost the nation an estimated $235 billion annually.[3]
- One in four children grow up in a household where alcohol is a problem.[4]
- Approximately 18 million people in the U.S. suffer from alcohol abuse or dependence.[5]
- Although individuals with alcohol use disorders (AUDs) who enter treatment are four times more likely to stop drinking, less than one quarter of those with AUDs actually receives treatment or participates in mutual or self-help groups.
- Excessive and/or chronic alcohol use may result in medical conditions such as liver and heart disease, pancreatitis, brain damage and esophageal and liver cancer.
- In 2007, alcoholic liver disease accounted for over 14,000 deaths and in 2008 was responsible for nearly 20% of liver transplants in the U.S.[6]
- Alcohol is the substance of choice among adolescents and is used by far more young people than tobacco or marijuana; early alcohol use is associated with future alcohol dependence.[7]
- Drinking during pregnancy can damage the developing embryo and fetus resulting in congenital organ defects and physical disabilities, as well as lifelong problems with cognition, behavioral control, and socialization.
[1] Mokdad AH, et al. JAMA. 2004. 29: 1238-45; CDC - Alcohol-Attributable Deaths Report, Average for United States 2001-2005.
[2] World Health Organization 2009. Global health risks: mortality and burden of disease attributable to selected major risks.
[3] Rehm J, et al. The Lancet 373(9682): 2223-2233, 27 June 2009-3 July 2009.
[4] Grant BF Am J Public Health. 2000. 90:112–115.
[5] Substance Abuse and Mental Health Services Administration. (2010). Results from the 2009 National Survey on Drug Use and Health: Vol I. Summary of National Findings (Office of Applied Studies, NSDUH Series H-38A, HHS Pub No. SMA 10-4586Findings).Rockville, MD.
[6] CDC - Deaths: Final Data for 2007, tables 10, 11, 13 - http://www.cdc.gov/NCHS/data/nvsr/nvsr58/nvsr58_19.pdf
[7] Op.cit. Substance Abuse and Mental Health Services Administration
NIAAA is Addressing Alcohol-related Problems Across the Lifespan
To reduce the tremendous burden of illness associated with alcohol misuse, NIAAA is working to prevent the onset and escalation of drinking during childhood and adolescence, and intervene for problem alcohol use at all ages. Support for research ranges from basic science to clinical studies and from genetics to neuroscience to epidemiology.
Enhancing the Evidence Base for Health Care Decisions
NIAAA is enhancing the evidence base for health care delivery for alcohol-related problems. Recognizing that screening and brief intervention (SBI) can efficiently and effectively be performed by primary care clinicians, NIAAA, with help from the American Medical Association, has put Helping Patients Who Drink Too Much: A Clinician’s Guide into the hands of over 250,000 physicians and nurses. The simple step of screening patients in primary care settings and providing brief behavioral interventions, and medications when indicated, has the potential to reduce health care costs significantly. In fact, SBI for alcohol misuse was ranked similarly in cost-effectiveness to screening for colorectal cancer and hypertension, and to influenza immunization.[8] Intervening for problem drinking prior to the onset of problems, or at early stages of dependence, reduces the need for more expensive, extensive inpatient treatment and also reduces societal costs of alcohol misuse including fatalities, injuries, violence, and lost work productivity. Making alcohol treatment available, accessible, affordable and perhaps most importantly, appealing, will increase the number of individuals who receive help before drinking destroys families, ruins careers, and costs society as a whole. NIAAA is supporting studies to expand the effective delivery of SBI to other venues and providers, investigate barriers to implementation, and capitalize on new technologies (see portrait).
To address the rampant problem of underage drinking across the nation, in 2011 NIAAA will release a developmentally appropriate alcohol screener and guide for pediatricians and other clinicians who provide medical care to children and adolescents. Screening sends the message that preventing alcohol use by their underage patients is as important to clinicians as ensuring that young people buckle their seatbelts and don their bicycle helmets. This empirically based screening instrument is devised to identify children at elevated risk for using alcohol as well as those children and adolescents who have already begun to experiment or are more heavily involved with alcohol. Protecting the developing body and brain from alcohol exposure is an important investment in short- and long-term health and builds on the success of screening for adults.
Initiative: Re-engineering the Therapeutic Development Pipeline
Quality healthcare also requires the availability of behavioral and pharmacotherapies that work, and knowledge of what works best for which patients. NIAAA has made considerable strides in re-engineering the therapeutic development pipeline, partnering with pharmaceutical companies, shortening the time required to test promising drugs, and improving the way alcohol clinical trials are conducted. In addition, by capitalizing on its long-term investment in studies on the genetics and neurocircuitry underlying alcohol dependence and the cellular mechanisms of tissue damage, NIAAA is successfully translating basic science to treatment both for alcohol dependence and alcohol related tissue and organ damage. NIAAA is also exploiting high throughput technologies for the development of new and more effective medications. For example, collection of genetic material as part of NIAAA’s National Epidemiologic Survey on Alcohol and Related Conditions, coupled with the ability to rapidly analyze vast amounts of genetic data, will not only enhance our understanding of the molecular mechanisms underlying alcohol dependence and co-occurring disorders and identify additional risk factors, but will also inform medications development and personalized treatment (see portrait). Coupling genetic information with alcohol clinical trials is already beginning to inform a personalized approach to treating alcohol dependence. Physicians will soon be able to prescribe medications based on a patient’s genetic make-up and drinking profile, thereby improving the success rate for treatment. Additional medications for liver disease are also on the horizon (see portrait). For example, preclinical studies of endocannabinoids have led to the development of new compounds that show promise for the reversal of early liver disease.[9] Interestingly, alcohol has also been shown to have beneficial cardiovascular effects. A recent high throughput screen identified a small molecule, Alda-1, that confers cardioprotection during cardiac ischemia by activating the alcohol-metabolizing enzyme ALDH2.[10],[11]
Overall Budget Policy:
Investigator-initiated research projects, new investigator research and research training are the Institute’s highest priorities. In FY 2012, NIAAA will support new investigators on R01 equivalent awards at success rates equivalent to those of established investigators submitting new R01 equivalent applications. Program plans in FY 2012 will support several other key initiatives of the NIH, including Enhancing the Evidence Base for Health Care Decisions and Re-engineering the Therapeutic Development Pipeline. Funds are included in the R&D contract mechanism to reflect the NIAAA share of trans-NIH initiatives, such as the Therapies for Rare and Neglected Diseases program (TRND), the Basic Behavioral and Social Sciences Opportunity Network (OppNet), and support for a new synchrotron at the Brookhaven National Laboratory. For example, each IC that will benefit from the new synchrotron will provide funding towards the NIH total $10.0 million commitment to support this technology.
[8] Solberg et al. Am J Prev Med 2008. 34: (2) 143-152.e3
[9] Tam J, Vemuri VK, Liu J, Bátkai S, Mukhopadhyay B, Godlewski G, Osei-Hyiaman D, Ohnuma S, Ambudkar SV, Pickel J, Makriyannis A, Kunos G. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010 Aug 2;120(8):2953-66. PMID: 20664173
[10] Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008 September 12; 321(5895):1493-1495.
[11] Perez-Miller, S., Younus, H., Vanam, R., Chen, C.H., Mochly-Rosen, D., Hurley, T.D. Alda-1 is an agonist and chemical chaperone for the common human aldehyde dehydrogenase 2 variant. Nat Struc Mol Biol. 2010 [Epub ahead of print 2010 Jan. 19]
Program Descriptions and Accomplishments
Embryo and Fetus:
The developing embryo and fetus are exquisitely vulnerable to the adverse effects of alcohol. NIAAA’s research support for this life stage encompasses outreach to pregnant women for identification and intervention of risky drinking; research to enhance our ability for early identification of and interventions with prenatal alcohol affected children; examination of nutritional and pharmacological agents that could lessen alcohol’s adverse effects on the developing embryo/fetus or reverse these effects in affected children; and research on how alcohol disrupts normal embryonic and fetal development. Research has shown that the severity of alcohol-related effects on the developing fetus is affected by the timing and level of maternal alcohol consumption, maternal nutritional status and maternal hormones. One of the key challenges facing clinicians is the ability to recognize women who are drinking in pregnancy and the infant who has been exposed to alcohol prenatally. Recent advances in methodologies for the measurement of alcohol metabolites which unlike the alcohol molecule itself, persist in various tissues such as blood, urine and hair for days to weeks have provided new opportunities for monitoring and assessing alcohol exposure. In addition, ongoing studies are demonstrating the utility of technologies including fetal ultrasound and 3D facial image analysis for earlier and improved recognition of children affected by prenatal alcohol exposure.
Budget Policy:
The FY 2012 budget estimate for this program is $20.507 million, an increase of $241.0 thousand or 1.2 percent over the comparable FY 2010 level. As a result of NIAAA’s affiliation with the scientific leadership in other countries, there is increasing recognition globally of the occurrence and devastating impact of fetal alcohol spectrum disorders. In FY 2012 NIAAA will enhance scientific collaboration with South Korea and Russia as well as continue to support research by U.S. investigators in South Africa where the prevalence of fetal alcohol spectrum disorders is especially high. Collectively, the focus of these activities is the identification of high risk drinking populations of women of childbearing age and the subsequent development and implementation of culturally appropriate interventions to prevent alcohol use during pregnancy.
Youth/Adolescence (Ages 0-17):
Adolescence is the time of life during which drinking, binge drinking (drinking five or more drinks on one occasion) and heavy drinking (binge drinking five or more times in the past 30 days) all ramp up dramatically. It is also a period of dramatic biological, social, and environmental changes. Given the pervasive use of alcohol among young people and the association between early initiation of alcohol use and future alcohol problems, NIAAA will release an alcohol screening guide in 2011 for health care providers to identify alcohol use, and alcohol use disorders in children and adolescents, and to identify risk for alcohol use, especially for younger children. NIAAA also has a significant research investment targeting this period of life. Studies focus on: 1) the effects of alcohol use on the developing body and brain, and 2) the interplay of development, genes, environment and adolescent alcohol use. During adolescence, the brain undergoes significant growth and remodeling raising concerns about alcohol’s effects on its development. Critical questions remain including: whether persistent changes in neural and behavioral function result from adolescent alcohol use; whether the same processes that confer adaptability of the adolescent brain to its environment also make it more vulnerable to alcohol-induced changes in structure and/or function; and whether such changes may increase an individual’s risk for future dependence. Recently completed pilot studies will be followed by additional research with human adolescents to determine if alcohol can disrupt, co-opt and/or alter normal developmental processes in the brain. NIAAA is also supporting a parallel initiative to study persistent alcohol-induced changes in the brain in animal models. Together these human and animal studies will set the stage for a larger scale initiative supporting longitudinal human studies to differentiate between the direct effects of alcohol and common underlying mechanisms in alcohol dependence, as well as to more fully assess other short- and long-term effects of alcohol exposure on the developing adolescent brain.
Budget Policy:
The FY 2012 budget estimate for this program is $61.504 million, an increase of $721.0 thousand or 1.2 percent over the comparable FY 2010 level. Building on the initial set of pilot projects to determine best practices for assessing the short and long-term impact of alcohol use on the developing adolescent brain, NIAAA anticipates supporting a second phase of pilot projects in FY 2012 that will inform a larger, future, longitudinal study. Complementing these human studies will be ongoing multidisciplinary animal studies focused on the persistent brain and behavioral functional changes following adolescent alcohol exposure including changes in gene expression, neurocircuitry and/or epigenetic modifications. The animal studies will also integrate the cellular, molecular, and genetic brain effects of adolescent alcohol exposure with emotional, social, cognitive, and behavioral development, including drinking behavior.
Young Adult (Ages 18-29):
For young adults, whose drinking behavior and extent of associated problems vary widely, NIAAA focuses on risk assessment, universal and selective prevention, and early intervention (before problems escalate and/or become chronic) and timely treatment for those who need it. Given the pervasiveness of high-risk drinking and alcohol dependence among young adults, efforts to alter drinking trajectories at this stage have life-changing potential and can significantly reduce the burden of illness resulting from alcohol-related problems. Recent research has demonstrated that college-aged individuals respond well to web-based screening and self-change programs, resulting in reductions in adverse alcohol-related consequences. NIAAA has released a free web-based consumer-oriented product, Rethinking Drinking, that can help people of all ages assess their own drinking, decide if they wish to change, and initiate and maintain change. This product may be particularly appealing and accessible to young adults. The Rethinking Drinking booklet is also a core component of a new, collaborative, national initiative to make alcohol screening and brief intervention a routine procedure in employee assistance programs (EAPs). To date, over 330,000 copies of the Rethinking Drinking booklet have been ordered by a wide variety of organizations including: EAPs; counseling centers; medical and mental health providers; health plans; Veterans Affairs facilities; high schools, colleges, and professional schools; and drug courts and DUI rehab programs. In addition, more than 415,000 individuals have visited the web site. For those who are unable to change on their own, NIAAA is focusing on uncovering the fundamental mechanisms underlying behavior change as well as assessing pharmacotherapies for younger people.
Budget Policy:
The FY 2012 budget estimate for this program is $174.315 million, an increase of $2.041 million or 1.2 percent over the comparable FY 2010 level. Studies using human alcoholic brains, gene knockout mice, and gene expression profiling have revealed an unambiguous link between alcohol exposure and altered neuroimmune function. However, it is essentially unknown how these neuroimmune molecules mediate brain functional changes due to alcohol exposure. Plans for FY 2012 include supporting research to study the neuroimmune response to alcohol and neuroimmune mechanisms underlying alcohol-related disorders. In addition, NIAAA will continue support for studies to expand alcohol screening and treatment for this population, including the use of new technologies, given the high prevalence of alcohol dependence among young adults.
Portrait of a Program: Using Evidence-based Research to Reform the Delivery of Healthcare
Funding levels:
FY 2010 Level: $13.562 million
FY 2012 Level: $13.779 million
Excessive alcohol use is a major contributor to the burden of disease and injury in the United States. Through support for basic and translational research, NIAAA has developed successful screening tools to identify individuals at risk for alcohol use disorders and methods clinicians can use to intervene with patients before problems escalate. This information is presented in the NIAAA’s Helping Patients Who Drink Too Much: A Clinician’s Guide, making it easier for health care providers to address a range of alcohol problems with their patients. However, there are still barriers to the widespread adoption of alcohol screening, brief intervention, and referral-to-treatment tools within general health care, and, in particular, primary health care. To address this issue, NIAAA is supporting studies focused on the effective implementation and delivery of these services. For example, Kaiser Permanente of Northern California, one of the largest health plans in the U.S. is conducting a study that incorporates the screening questions from NIAAA’s Clinician’s Guide into the health care provider’s “visit navigator”, an electronic decision tool that guides primary care physicians through their workflow during patient visits. The integrated workflow and electronic medical records system are then used to evaluate whether physicians became more proficient at identifying at-risk patients and whether they prescribed appropriate medications for alcohol dependence as well as other psychiatric conditions such as depression. Improvements in short- and long-term health outcomes for patients and trends in cost per patient treated are also being evaluated. Improving the quality of specialty care for individuals with co-occurring psychiatric and alcohol use disorders is also an NIAAA priority. In one study, data collected from over 400 alcohol treatment centers across the U.S. are being examined to determine the organizational and economic barriers to the implementation of more integrated and evidence-based treatment methods. Studies that compare the effectiveness of different treatments in real world settings are also being supported by NIAAA. Such studies can inform decision making by patients and their health care providers, and lead to adoption of more cost-effective treatments. Finally, the explosion in mobile health technology has created new opportunities for research. NIAAA is capitalizing on this new technology including supporting a study on interventions that can be targeted to individuals on a location-specific basis using GPS-enabled mobile phones and text messaging. Ultimately, the goal of research is to improve the health of the nation. NIAAA continues to work with other HHS agencies, including the Substance Abuse and Mental Health Services Administration (SAMHSA), the Health Resources and Services Administration (HRSA), and the Indian Health Service (IHS) to address the larger goal of linking research findings, primary and preventive care, and community prevention services.
Midlife/Senior Adult:
Research has demonstrated that there is no typical alcoholic; the variation among individuals who meet criteria for alcohol dependence reflects both the subtype of dependence and individual genetic make-up. NIAAA’s research focus for the midlife/senior population encompasses: 1) identification of mechanisms by which alcohol and its metabolites cause tissue and organ pathologies; 2) development of treatment strategies for alcohol dependence (including medications) that are tailored to specific populations; and 3) treatment of individuals with co-existing psychiatric and medical disorders. In its ongoing efforts to deal with one of the most serious medical consequences of alcohol dependence, NIAAA continues to support medications development for the treatment of alcoholic liver disease and continues to seek biomarkers for liver damage. In order to better understand how alcohol causes cell and tissue damage in multiple organs in the body, NIAAA supports studies using a systems biology approach to investigate how pathological changes in one organ can also result in physiological aberrations in another. The interactions between the gut, liver and brain are a focus of such an approach. To ensure efficient testing of promising compounds for alcohol dependence and to move them more quickly through early clinical testing, the Institute established the NIAAA Clinical Investigations Group. By conducting early clinical trials itself, NIAAA anticipates that pharmaceutical companies will be more willing to pursue those compounds shown to be effective. In its medications development program, NIAAA has been especially successful at linking individual genetic variation with positive outcomes for specific medications.
Budget Policy:
The FY 2012 budget estimate for this program is $133.923 million, an increase of $1.569 million or 1.2 percent over the comparable FY 2010 level. In FY 2012, the focus for this program will continue to be the development of treatment for alcohol dependence and the other consequences of harmful drinking, especially on medications development. Support for studies to develop pharmacological and behavioral treatments for individuals who have alcohol use disorders, including those with co-existing other drug, psychiatric and/or physical disorders continues to be a priority. In addition, NIAAA will support studies that compare the effectiveness of various treatments.
Portrait of a Program: Progress in Pharmacotherapy for Alcohol Dependence and Alcoholic Liver Disease
FY 2010 Level: $34.731 million
FY 2012 Level: $35.287 million
The goal of the NIAAA medications development program is to develop safe, effective medications to treat the medical consequences of heavy alcohol use including alcohol dependence, and tissue and organ damage throughout the body. The focus on compounds to treat alcohol dependence has lead to a number of potential medications and development of new pharmacotherapies continues to be a top priority for NIAAA. The Institute recently conducted Phase 2 clinical trials of two FDA-approved medications, the mood stabilizing drug quetiapine and the antiepileptic drug levetiracetam, for promoting either abstinence or reduction in heavy drinking. In FY 2011, Phase 2 studies will be initiated for varenicline, a smoking cessation medication that has shown promising preliminary results in reducing alcohol consumption. NIAAA is also exploring genetic variations that predict positive drinking outcomes in individuals in response to odansetron (which blocks the action of serotonin) and naltrexone (FDA approved for alcohol dependence), helping to make personalized medicine for alcohol dependence a reality. The consequences of heavy chronic drinking extend beyond alcohol dependence and include tissue and organ damage as well. Alcoholic liver disease (ALD) is a potentially life-threatening disease that without proper treatment may progress to liver cirrhosis, liver cancer or liver failure. Liver transplantation is often the only viable option for advanced liver disease but it is a prolonged, expensive and risky process only available to patients who maintain abstinence. Steroids are commonly prescribed as a palliative measure for liver disease but may have serious side effects. Safer medications that can slow or even reverse progression to advanced liver disease are urgently needed. NIAAA is pursuing several promising compounds for treatment of ALD. For example, refanalin, is currently in Phase I testing for reversal of liver fibrosis. This clinical trial is the result of a joint venture of NIAAA and NIDDK. The dietary supplement S-adenosylmethionine (SAMe), as shown in a recent Phase 2 trial, may be effective in preventing early liver disease and even later stage liver cancer. Zinc, another dietary supplement, has shown promise in animal studies and is being pursued for potential testing in humans. In FY 2010, NIAAA demonstrated that an inhibitor of the endocannabinoid CB1 receptor promoted weight loss in obese mice and improved obesity-related metabolic complications, such as fatty liver, a precursor to advanced liver disease, and diabetes. Toxicology studies of a similar inhibitor are anticipated and could soon lead to human trials. Clinical trials of medications, including pentoxifylline, that have shown promise in off label use for liver disease and have fewer undesirable side effects are also anticipated. As medications are developed and tested, NIAAA will continue to explore ways to make testing more efficient; collaborate with the pharmaceutical industry to test novel compounds; and collaborate with the FDA and other entities to improve guidelines and methodology for alcohol clinical trials.
Intramural Research:
The Intramural Research Program has made significant advances in the areas of medications development, neuroscience, genetics, epidemiology and physiology. A major focus of NIAAA in general, and of the Intramural Program specifically, has been to improve treatment of alcohol use disorders and associated problems. The approach of identifying molecular targets for treatment in experimental animals and then validating these targets in clinical research studies has been successful. There has also been considerable progress in identifying genes that underlie alcohol dependence and that influence the efficacy of specific treatments, confirming that some treatments are more effective than others in individuals with particular gene variants. The Intramural Research Program also focuses on understanding and preventing alcohol-related liver disease. Studies have revealed that endocannabinoids – endogenous marijuana-like substances – are necessary in a specific type of liver cell for the development of alcohol-induced fatty liver, a forerunner of more serious liver diseases, such as cirrhosis and liver cancer. Other studies have demonstrated that dietary supplements can prevent the development of alcohol-induced fatty liver and mitochondrial dysfunction in animal models. In addition, the Intramural Program continues to conduct large scale studies that provide information on the extent of alcohol and other drug dependence and co-occurring mental disorders in the U.S. population. Treatment studies on alcohol dependence and co-occurring psychiatric disorders are also underway.
Budget Policy: The FY 2012 budget estimate for this program is $49.376 million, an increase of $1.588 million or 3.3 percent over the comparable FY 2010 level. The increase will be used to offset expenses associated with the January 2010 pay raise and other increases that will allow NIAAA continued support for its laboratories within its Division of Intramural Clinical and Biological Research as well as the Intramural Office of Laboratory Animal Science.
Research Management and Support:
NIAAA RMS activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. RMS functions also encompass strategic planning, coordination, and evaluation of the Institute’s programs, regulatory compliance, international coordination, and liaison with other Federal agencies, Congress, and the public.
Budget Policy:
The FY 2012 budget estimate for this program is $29.572 million, an increase of $951.0 thousand or 3.3 percent over the comparable FY 2010 level. The increase will be used to offset expenses associated with the January 2010 pay raise and other mandatory cost increases necessary to provide for the effective administration, planning and evaluation, public information and communication, and scientific leadership of the institute.
NIH Common Fund
The NIAAA participates in the support of the Interdisciplinary Research initiative funded through the NIH Common Fund. NIAAA participates in the NIH mouse knockout project, the ultimate goal of which is to produce and characterize a library of mouse lines containing mutations that abolish the function of each of 8500 genes. This will comprise part of a larger collaborative effort which will produce mouse lines with mutations in every gene in the mouse genome. Studies capitalizing on the ability to delete individual genes have elucidated the roles of thousands of genes in mammalian embryonic development, adult physiology, aging, and disease. Expanding the availability of mouse mutants to include deletions of every gene in the genome will enhance understanding of human traits and disease processes. NIAAA also participates in the NIH Science of Behavior Change program the goal of which is to improve the understanding of basic mechanisms of behavior change that play a role in initiating or maintaining behavior change across a broad range of health-related behaviors.
NATIONAL INSTITUTES OF HEALTH
National Institute on Alcohol Abuse and Alcoholism
Budget Authority by Object Class
(Dollars in Thousands)
|
FY 2010 Actual |
FY 2012 PB |
Increase or Decrease |
Percent Change |
|
|---|---|---|---|---|
|
Total Budget Authority by Object |
$462,086 |
$469,197 |
$7,111 |
1.5% |
|
Personnel Compensation: |
||||
| 11.1 Full-time permanent | $17,394 | $17,433 | $39 | 0.2% |
| 11.3 Other than full-time permanent | 6,487 | 6,502 | 15 | 0.2% |
| 11.5 Other personnel compensation | 792 | 793 | 1 | 0.1% |
| 11.7 Military personnel | 501 | 517 | 16 | 3.2% |
| 11.8 Special personnel services payments | 3,208 | 3,439 | 231 | 7.2% |
|
Total, Personnel Compensation |
$28,382 |
$28,684 |
$302 |
1.1% |
|
Subtotal, Non-Pay Costs |
$426,517 |
$433,311 |
$6,794 |
1.6% |
|
12.0 Personnel benefits |
$6,692 |
$6,706 |
$14 |
0.2% |
| 12.2 Military personnel benefits | 495 | 496 | 1 | 0.2% |
| 13.0 Benefits for former personnel | 0 | 0 | 0 | 0.0% |
|
Subtotal, Pay Costs |
$35,569 |
$35,886 |
$317 |
0.9% |
|
21.0 Travel and transportation of persons |
$971 |
$971 |
$0 |
0.0% |
|
22.0 Transportation of things |
49 |
54 |
5 |
10.2% |
|
23.1 Rental payments to GSA |
1 |
1 |
0 |
0.0% |
|
23.2 Rental payments to others |
9 | 10 | 1 | 11.1% |
|
23.3 Communications, utilities and miscellaneous charges |
311 | 344 | 33 | 10.6% |
|
24.0 Printing and reproduction |
177 | 201 | 24 | 13.6% |
|
25.1 Consulting services |
299 | 321 | 22 | 7.4% |
|
25.2 Other services |
5,864 | 6,541 | 677 | 11.5% |
|
25.3 Purchase of goods and services from government accounts |
44,208 | 49,363 | 5,155 | 11.7% |
|
25.4 Operation and maintenance of facilities |
53 | 59 | 6 | 11.3% |
|
25.5 Research and development contracts |
17,629 | 19,445 | 1,816 | 10.3% |
|
25.6 Medical care |
116 | 126 | 10 | 8.6% |
|
25.7 Operation and maintenance of equipment |
706 | 779 | 73 | 10.3% |
| 25.8 Subsistence and support of persons | 0 | 0 | 0 | 0.0% |
|
Total compensable workyears: |
||||
| Full-time employment | 224 | 224 | 0 | 0.0% |
| Full-time equivalent of overtime and holiday hours | 1 | 1 | 0 | 0.0% |
| Average ES salary | $179,700 | $183,675 | $3,975 | 2.2% |
| Average GM/GS grade | 12.7 | 12.7 | 0.0 | 0.0% |
| Average GM/GS salary | $104,972 | $107,294 | $2,322 | 2.2% |
| Average salary, grade established by act of July 1, 1944 (42 U.S.C. 207) | $134,138 | $135,614 | $1,476 | 1.1% |
| Average salary of ungraded positions | 127,044 | 128,442 | 1,398 | 1.1% |
|
OBJECT CLASSES |
FY 2010 Actual |
FY 2012 Estimate |
Increase or Decrease |
Percent Change |
|
26.0 Supplies and materials |
$3,910 |
$4,167 |
$257 |
6.6% |
| 31.0 Equipment | 2,276 | 2,495 | 219 | 9.6% |
| 32.0 Land and structures | 0 | 0 | 0 | 0.0% |
| 33.0 Investments and loans | 0 | 0 | 0 | 0.0% |
| 41.0 Grants, subsidies and contributions | 349,937 | 348,433 | (1,504) | -0.4% |
| 42.0 Insurance claims and indemnities | 0 | 0 | 0 | 0.0% |
| 43.0 Interest and dividends | 1 | 1 | 0 | 0.0% |
| 44.0 Refunds | 0 | 0 | 0 | 0.0% |
|
25.0 Subtotal, Other Contractual Services |
$68,875 |
$76,634 |
$7,759 |
11.3% |
NATIONAL INSTITUTES OF HEALTH
National Institute on Alcohol Abuse and Alcoholism
Salaries and Expenses
(Dollars in Thousands)
|
OBJECT CLASSES |
FY 2010 Actual |
FY 2012 PB |
Increase or Decrease |
Percent Change |
|---|---|---|---|---|
|
Personnel Compensation: |
||||
| Full-time permanent (11.1) | $17,394 | $17,433 | $39 | 0.2% |
| Other than full-time permanent (11.3) | 6,487 | 6,502 | 15 | 0.2% |
| Other personnel compensation (11.5) | 792 | 793 | 1 | 0.1% |
| Military personnel (11.7) | 501 | 517 | 16 | 3.2% |
| Special personnel services payments (11.8) | 3,208 | 3,439 | 231 | 7.2% |
|
Total Personnel Compensation (11.9) |
$28,382 |
$28,684 |
$302 |
1.1% |
|
Civilian personnel benefits (12.1) |
$6,692 |
$6,706 |
$14 |
0.2% |
| Military personnel benefits (12.2) | 495 | 496 | 1 | 0.2% |
| Benefits to former personnel (13.0) | 0 | 0 | 0 | 0.0% |
|
Subtotal, Non-Pay Costs |
$42,769 |
$44,954 |
$2,185 |
5.1% |
|
Travel (21.0) |
$971 |
$971 |
$0 |
0.0% |
| Transportation of things (22.0) | 49 | 54 | 5 | 10.2% |
| Rental payments to others (23.2) | 9 | 10 | 1 | 11.1% |
| Communications, utilities and miscellaneous charges (23.3) | 311 | 344 | 33 | 10.6% |
| Printing and reproduction (24.0) | 177 | 201 | 24 | 13.6% |
| Other Contractual Services: | ||||
| Advisory and assistance services (25.1) | 299 | 321 | 22 | 7.4% |
| Other services (25.2) | 5,864 | 6,541 | 677 | 11.5% |
| Purchases from government accounts (25.3) | 30,423 | 31,510 | 1,087 | 3.6% |
| Operation and maintenance of facilities (25.4) | 53 | 59 | 6 | 11.3% |
| Operation and maintenance of equipment (25.7) | 706 | 779 | 73 | 10.3% |
| Subsistence and support of persons (25.8) | 0 | 0 | 0 | 0.0% |
|
Subtotal Other Contractual Services |
$37,345 |
$39,210 |
$1,865 |
5.0% |
|
Supplies and materials (26.0) |
$3,907 |
$4,164 |
$257 |
6.6% |
| Total, Administrative Costs | $78,338 | $80,840 | $2,502 | 3.2% |
|
Subtotal, Pay Costs |
$35,569 |
$35,886 |
$317 |
0.9% |
NATIONAL INSTITUTES OF HEALTH
National Institute on Alcohol Abuse and Alcoholism
Details of Full-Time Equivalent Employment (FTEs)
|
OFFICE/DIVISION |
FY 2010 Actual |
FY 2011 CR |
FY 2012 PB |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
Civilian |
Military |
Total |
Civilian |
Military |
Total |
Civilian |
Military |
Total |
|
|
Office of the Director |
8 |
8 |
8 |
8 |
8 |
|
8 | ||
| Office of Extramural Activities | 14 | 14 | 14 | 14 | 14 | 14 | |||
| Office of Science Policy and Communications | 16 | 16 | 16 | 16 | 16 | 16 | |||
| Division of Epidemiology and Prevention | 34 | 34 | 34 | 34 | 34 | 34 | |||
| Research | 11 | 11 | 11 | 11 | 11 | 11 | |||
| Division of Metabolism and Health Effects | 11 | 11 | 11 | 11 | 11 | 11 | |||
| Division of Neuroscience and Behavior | 14 | 14 | 14 | 14 | 14 | 14 | |||
| Division of Treatment Recovery Research | 8 | 1 | 9 | 8 | 1 | 9 | 8 | 1 | 9 |
| Division of Intramural Research Programs | 103 | 4 | 107 | 103 | 4 | 107 | 103 | 4 | 107 |
|
Total |
219 |
5 |
224 |
219 |
5 |
224 |
219 |
5 |
224 |
|
Includes FTEs which are reimbursed from the NIH Common Fund for Medical Research |
|||||||||
|
FISCAL YEAR |
Average GS Grade |
||||||||
|
2008 |
12.5 |
||||||||
| 2009 | 12.6 | ||||||||
| 2010 | 12.7 | ||||||||
| 2011 | 12.7 | ||||||||
| 2012 | 12.7 |
NATIONAL INSTITUTES OF HEALTH
National Institute on Alcohol Abuse and Alcoholism
Detail of Positions
|
GRADE |
FY 2010 Actual |
FY 2011 CR |
FY 2012 PB |
|---|---|---|---|
|
Grades established by Act of July 1, 1944 (42 U.S.C. 207): |
|||
| Assistant Surgeon General | 0 | 0 | 0 |
| Director Grade | 3 | 3 | 3 |
| Senior Grade | 1 | 1 | 1 |
| Full Grade | 0 | 0 | 0 |
| Senior Assistant Grade | 0 | 0 | 0 |
| Assistant Grade | 0 | 0 | 0 |
|
Subtotal |
4 |
4 |
4 |
| Ungraded | 76 | 76 | 76 |
| Total permanent positions | 170 | 170 | 170 |
| Total positions, end of year | 246 | 246 | 246 |
| Total full-time equivalent (FTE) employment, end of year | 224 | 224 | 224 |
| Average ES salary | 179,700 | 181,677 | 183,675 |
| Average GM/GS grade | 12.7 | 12.7 | 12.7 |
| Average GM/GS salary | 104,972 | 106,127 | 107,294 |
|
Total, ES Positions |
1 |
1 |
1 |
| Total, ES Salary | 179,700 | 181,677 | 183,675 |
|
GM/GS-15 |
22 |
22 |
22 |
| GM/GS-14 | 45 | 45 | 45 |
| GM/GS-13 | 41 | 41 | 41 |
| GS-12 | 29 | 29 | 29 |
| GS-11 | 10 | 10 | 10 |
| GS-10 | 3 | 3 | 3 |
| GS-9 | 7 | 7 | 7 |
| GS-8 | 3 | 3 | 3 |
| GS-7 | 3 | 3 | 3 |
| GS-6 | 1 | 1 | 1 |
| GS-5 | 1 | 1 | 1 |
| GS-4 | 0 | 0 | 0 |
| GS-3 | 0 | 0 | 0 |
| GS-2 | 0 | 0 | 0 |
| GS-1 | 0 | 0 | 0 |
|
Subtotal |
165 |
165 |
165 |

