Goal 4: Treatment
Develop and Improve Treatments for Alcohol Misuse, Alcohol Use Disorder, Co-Occurring Conditions, and Alcohol-Related Consequences

Considering the serious adverse consequences associated with alcohol misuse noted above, treating alcohol use disorder (AUD) and ameliorating the negative health effects associated with it are crucial. A variety of effective interventions are available to treat AUD. These include professionally led behavioral treatments to help people identify and change behaviors that contribute to alcohol misuse, three U.S. Food and Drug Administration (FDA)–approved medications, and mutual support groups, such as Alcoholics Anonymous and other peer-led groups. Research shows that behavioral and pharmacological therapies are most effective when used in combination, and that most people with AUD who get some form of treatment benefit from it.
Unfortunately, however, many of the people who might benefit from alcohol treatment never receive it: less than 10 percent of people in the United States who had AUD in the past year received treatment or help,36 and less than 4 percent of those who had AUD in the past year received an FDA-approved alcohol treatment medication.37 Numerous barriers prevent individuals from seeking treatment. They may be deterred by the stigma associated with alcohol misuse and alcohol-related disease, financial constraints, or logistical challenges that prevent them from attending treatment, such as lack of transportation or childcare. Still others, including many clinicians, are not aware of the full range of alcohol treatment options. The barriers to seeking and using preventive and treatment services—and the outcomes of those services—are not necessarily uniform across demographic groups. Improving the identification of high-risk alcohol users and expanding access to a diverse, high-quality, and culturally and age-appropriate range of health services is critical to reducing the public health burden of alcohol misuse.
Treatment for alcohol misuse has largely been administered in specialty treatment facilities. However, the quality and accessibility of care could be improved by integrating alcohol services into general medical care. Primary care settings, for example, provide opportunities for regular screening, engaging patients who may be unwilling or unable to seek specialty care, treating the physical and mental health conditions that commonly co-occur with AUD, and monitoring recovery. Integrated care models, facilitated by electronic medical records, are beginning to emerge, but these models and approaches for implementing and sustaining them must be evaluated.
Mobile health technologies hold promise for improving upon and expanding access to evidence-based interventions for problem drinking. Smart phones, text messaging, social media applications, biological sensors, and other devices are being used to engage patients outside of usual care settings, assist them in monitoring their alcohol consumption, and provide personalized resources and support where and when they need it most, such as at times or places they tend to use alcohol. (See “NIAAA Wearable Alcohol Biosensor Challenge.”) Although there are significant challenges to optimizing and facilitating uptake of electronic health technologies, research in this area has the potential to increase the number of people with access to effective, personalized treatment interventions and continuing care.
NIAAA will continue to pave a path to healthier lives for the millions of people affected by alcohol-related disease by supporting a diverse portfolio of treatment and recovery research, with a focus on the following objectives:
Objective 4a: Improve existing behavioral treatments for alcohol use disorder and co-occurring conditions, and develop new behavioral treatments based on advances in neuroscience and basic behavioral research
Cognitive behavioral therapy, motivational therapy, community reinforcement, family and couples therapy, and brief interventions have consistently been found to reduce rates of heavy drinking. Such behavioral treatments for AUD share features, such as having health care professionals work with people to set attainable alcohol reduction goals, build a strong social support system, and develop skills to cope with or avoid triggers that might contribute to relapse. Behavioral treatments also differ in certain ways. For example, cognitive behavioral therapy is aimed at changing the thought processes that contribute to drinking and teaching coping skills directed at managing risky situations. Motivational enhancement therapy, on the other hand, seeks to facilitate and strengthen internal motivation to change drinking behavior by aligning changes in behavior with life goals.
As with pharmacological treatments, particular behavioral strategies are not effective for every patient, and individuals diagnosed with AUD and co-occurring psychiatric conditions often have poorer treatment outcomes. Developing a better understanding of mechanisms through which behavioral interventions exert their effects (i.e., the active ingredients of these therapeutic approaches) will enable health care providers to optimize care by emphasizing the treatment features that have the greatest and longest-lasting impact on outcomes. Research has already identified several possible psychological mechanisms, including eliciting change/commitment talk, therapeutic alliance, client self-efficacy, therapist empathy, coping skills, motivation to change, and attributions of change. Researchers are also examining how patterns of brain activity may be used to predict treatment outcomes, and even whether altering activity in brain networks involved in AUD can influence drinking. Additional research is needed to identify novel mechanisms of behavior change, further elucidate those that have empirical support, and translate this knowledge into new and improved treatments.
To advance behavioral treatments for AUD and co-occurring conditions, NIAAA will:
- Promote research to enhance the effectiveness of current, evidence-based behavioral therapies for a wider range of patients across a wider range of treatment settings.
- Examine the effectiveness of novel behavioral interventions for AUD and co-occurring psychiatric conditions, such as interventions that target reward systems, emotional regulation, stress responsivity, cognitive function, and sleep.
- Characterize changes in neural circuits that accompany responses to behavioral treatments for AUD, use this information to optimize and validate AUD interventions, and determine which type of behavioral therapy works best for which patient.
- Investigate the use of noninvasive devices capable of altering brain activity, such as transcranial direct current stimulation, as AUD treatments or adjuncts to treatment.
- Use electronic health technologies, including mobile devices, to improve the effectiveness, accessibility, and use of behavioral interventions for AUD and co-occurring disorders, including human immunodeficiency virus (HIV).
Objective 4b: Develop novel medications for treating alcohol use disorder and co-occurring conditions
Medications are an important component of the AUD treatment toolbox, and they are often used in combination with behavioral interventions for AUD. There are currently three medications approved by the FDA for treating AUD: disulfiram, naltrexone, and acamprosate. Disulfiram blocks an enzyme in the body involved in metabolizing acetaldehyde, a toxic byproduct of alcohol metabolism. It causes unpleasant side effects such as nausea, vomiting, and flushing of the skin when alcohol is consumed, which helps to deter people who are taking disulfiram from drinking. Naltrexone, which is available in oral and extended-release injectable formulations, diminishes the rewarding effects of alcohol to help reduce heavy drinking. Acamprosate reduces the negative emotional state associated with protracted abstinence from alcohol and may also reduce craving, making it easier to maintain abstinence once it is achieved.
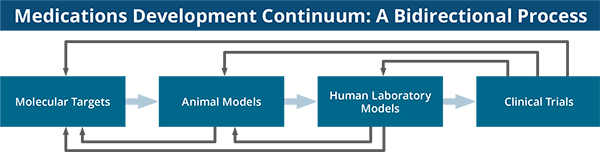
Additional AUD treatments are needed, including for people with co-occurring health conditions, such as post-traumatic stress disorder (PTSD), HIV, and alcoholic liver disease (ALD), as well as other substance use disorders. Research is also needed on the safety and efficacy of AUD medications in adolescents, who tend not to be represented in clinical trials. Through its intramural and extramural research programs, NIAAA supports studies across the drug development continuum, from target identification to clinical trials, with the goals of developing a wider variety of safe and effective pharmacotherapies and giving clinicians a menu of options for individualized AUD treatment. (See “Medications Development Continuum: A Bidirectional Process. ”) At present, researchers have identified more than 30 molecular targets that appear to alter drinking behavior. However, much work is needed to determine the precise role of these targets in the development and maintenance of AUD. Once a promising target has been selected, it is necessary to identify and screen compounds capable of modulating it. Only a small percentage of candidate compounds are ever approved for use. In fact, to take a central nervous system compound from discovery to market takes approximately 18 years and more than $1.8 billion.38, 39 To make this process more efficient and less costly, NIAAA is pursuing new approaches to identify drug targets using network biology analyses to examine relationships among candidate compounds, gene expression profiles, and protein and metabolite products associated with AUD. Such approaches reflect a shift toward developing compounds or combinations of compounds that can simultaneously modulate multiple biological targets with a role in AUD. The Institute also continues to support the development and validation of standardized animal screening models with which to evaluate the preclinical efficacy of compounds.
Another particularly challenging barrier to medications development is moving promising compounds from animal studies into human testing. NIAAA is working to bridge the gap between basic and clinical research via its Human Laboratory Program (HLAB) and through the Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) programs. Through the HLAB, NIAAA aims to standardize clinical laboratory paradigms for screening experimental AUD medications for efficacy and safety. Under the SBIR and STTR programs, NIAAA assists small businesses with early-stage compounds to conduct pre-Investigational New Drug (IND) studies intended to result in the submission of an IND application to the FDA. This program also supports Phase I clinical studies to ensure the safety, tolerability, and proper dosing of compounds approved for experimental use in people.
Although human laboratory studies can provide preliminary evidence for a compound’s efficacy, randomized, placebo-controlled, Phase II proof-of-concept (POC) trials are required to validate these findings. NIAAA has a long history of supporting state-of-the-art POC trials of promising AUD medications, including trials in AUD patients with co-occurring conditions. To expedite such studies, NIAAA’s Clinical Investigations Group (NCIG) uses a network of sites to conduct “fast success/fast fail” trials in less than two years in collaboration with the pharmaceutical industry and the National Center for Advancing Translational Sciences.
NIAAA seeks to engage and partner with the pharmaceutical industry to conduct Phase III (pivotal trials) on compounds with promising POC data. NIAAA also supports secondary analyses of trial data that can inform regulatory decision-making and the development of evidence-based research guidelines. For example, studies supported by NIAAA led the FDA to allow “percent of subjects with no heavy drinking days” to be used as an alternative to abstinence as the primary endpoint in trials of drugs evaluated as AUD treatments. NIAAA hopes these efforts will further stimulate the development of treatments that provide meaningful clinical benefits to patients.
NIAAA will continue to prioritize the development of a wider array of medications to treat individuals diagnosed with AUD alone or with other diseases and conditions. To further this goal, NIAAA will support research to:
- Explore, with secondary analyses and in consultation with the FDA, new, clinically meaningful endpoints for clinical trials of medications for treating AUD.
- Use new approaches, such as drug-target networks and connectivity mapping, to expedite the identification of neurobiological drug targets involved in each stage of the AUD cycle, and to identify and optimize compounds that act on those targets as potential treatments for AUD.
- Continue to develop human laboratory paradigms as screening models to test compounds for treating AUD.
- Conduct clinical trials on novel and repurposed compounds with potential for treating AUD.
- Reverse-validate preclinical models and human laboratory models with effective medications as they are developed.
- Identify and evaluate candidate therapeutics for treating AUD in patients with co-occurring mental health conditions, alcohol-related organ damage, and HIV, as well as in adolescents.
Objective 4c: Identify factors that facilitate or inhibit sustained recovery from alcohol use disorder
Though definitions vary, one conceptualization of recovery from AUD is the disappearance of AUD symptoms accompanied by a state of well-being that builds resilience to relapse. Recovery is possible and is associated with parallel neuropsychological and neurobiological changes. However, the process is not the same for everyone. Some people need longer or more intense treatments, whereas others recover more quickly and with minimal intervention. Relapse is often a part of the process; it may take several attempts before someone can stop or reduce drinking over the longer term. A return to drinking after a period of abstinence is especially likely during times of stress or when individuals are exposed to people or places they associate with drinking. Currently, little is known about the factors that facilitate or inhibit long-term recovery, including why some people can recover without some form of assistance. Developing a better understanding of the recovery process, including the factors that enable people to maintain changes in their drinking behavior and promote resilience to relapse, will inform the development of additional effective treatment interventions.
To further this goal, NIAAA will:
- Collaborate with stakeholders to develop operational definitions of recovery and relapse.
- Support observational, epidemiological, and natural history studies that lay the foundation for understanding mediators and moderators of recovery, such as stress and peer and family support, in subgroups of individuals with AUD.
- Support research on the use of biomarkers and behavioral and neuropsychological assessments validated by neuroimaging to monitor the recovery process and inform follow-up intervention. (See also Objective 1a.)
- Evaluate the effectiveness of the full range of recovery support services, including those that are particularly successful at recruiting understudied populations, such as women and racial and ethnic minorities.
- Explore the optimal timing and dose of AUD interventions, including pharmacotherapies, behavioral treatments, and mutual help groups, needed to maximize and sustain recovery.
Objective 4d: Advance precision medicine by evaluating which treatments for alcohol use disorder and related conditions work best for which individuals
Precision medicine is an emerging approach for preventing and treating disease that considers individual variability in genes, environment, and lifestyle. It is an important focus of the NIH and a guiding framework for NIAAA’s work to treat alcohol misuse and AUD. NIAAA’s Addictions Neuroclinical Assessment will provide a framework for diagnosing AUD based on neurobiological, behavioral, and genetic phenotypes. (See “Addictions Neuroclinical Assessment.”) NIAAA also supports research to identify patient cohorts most likely to benefit from existing treatments and to develop new interventions based on the unique characteristics of a person’s disease. For example, because relapse is frequently triggered by stress, NIAAA is evaluating compounds that target brain stress systems as treatments for people who are particularly susceptible to stress-related drinking. Researchers are also investigating whether patterns of brain activation can be used to identify people who are likely to relapse when exposed to certain stimuli, as well as whether particular AUD treatments are more effective in people with certain gene variants.
Identifying subpopulations that are most likely to respond to certain treatments may help augment the effect sizes found in studies of candidate AUD medications. So far, effect sizes for compounds that act on the central nervous system for conditions such as addiction, depression, and schizophrenia have tended to be modest. Improving effect sizes may encourage greater pharmaceutical interest in developing AUD medications and expedite the production of a broader menu of effective treatments. Together, these efforts hold promise for a future in which clinicians will be able to use brain imaging, genetic testing, biomarker profiling, psychological assessments, and other diagnostic techniques to identify patients most likely to benefit from a given therapy and monitor their progress over the course of treatment and recovery.
To fulfill the promise that precision medicine holds for improving AUD treatment, NIAAA will pursue research and related initiatives to:
- Identify and evaluate biological, cognitive, and behavioral markers that can be used to predict a patient’s response to AUD treatments and treatment combinations, and evaluate their progress throughout recovery.
- Identify which preventive and treatment interventions work best for whom by creating DNA, RNA, and protein databases with samples from NIAAA research participants and making these data available to the broader research community.
Objective 4e: Develop and evaluate interventions to treat fetal alcohol spectrum disorders, alcoholic liver disease, and other negative health outcomes caused by alcohol misuse
Fetal alcohol spectrum disorders (FASD) are among the many devastating negative health outcomes that can result from alcohol misuse. Although there is no cure for FASD, there are interventions that can improve outcomes for individuals affected by these conditions. For example, school-based interventions, which include specialized teaching strategies that provide a consistent routine and numerous opportunities to practice new skills, can help children learn more effectively. Researchers are investigating other approaches to improving cognitive and behavioral function in people with FASD, including game-based interventions to improve attention and working memory; training in metacognition, literacy, problem solving, social skills, and personal safety; and pre- and postnatal nutritional supplements. The use of family support groups and classes to help parents better care for children with FASD are also being investigated as potential strategies for improving behavioral outcomes in affected children.
NIAAA will continue to support research to mitigate the developmental, cognitive, and behavioral challenges faced by people with FASD, including studies to:
- Identify and evaluate nutritional and pharmacological agents that could lessen alcohol’s adverse effects on prenatal development or ameliorate these effects after birth.
- Develop and evaluate novel, developmentally relevant behavioral interventions to lessen the sensory, motor, cognitive, and emotional challenges experienced by children and adults with FASD.
Alcoholic liver disease (ALD) is a serious and potentially fatal consequence of alcohol misuse for which there is a dire need for new treatments. Advanced stages of ALD are very difficult to treat, and many patients do not respond to the pharmacotherapies that are currently available. Indeed, more than 50 percent of patients with a severe case of alcoholic hepatitis die within the first month of being diagnosed.20 NIAAA has a major ongoing initiative to fund multiple translational research consortia dedicated to developing more effective treatments for alcoholic hepatitis.
Researchers have made advances in understanding the mechanisms by which alcohol misuse leads to liver fibrosis and cirrhosis. This is opening new avenues for the investigation of compounds to reverse these conditions, which were previously considered untreatable. There is growing optimism that compounds that target multiple pathological mechanisms responsible for ALD may provide better clinical outcomes than current treatments. Additional research is needed to capitalize on these advances and ensure continued progress against ALD. (See Objective 4b to learn more about NIAAA’s work in medications development.) The use of liver transplantation in ALD patients is also an important area of inquiry. Although this procedure is the only effective treatment for end-stage liver disease, only a minority of ALD patients are referred for liver transplant, and there is insufficient information on clinical outcomes of liver transplantation in this population.
To improve treatment outcomes for patients with ALD, NIAAA will support research and related activities to
- Identify and evaluate novel nutritional and pharmacological agents for treating ALD.
- Develop and integrate pharmacological and behavioral AUD treatments for individuals undergoing ALD treatment.
Objective 4f: Evaluate the effectiveness, accessibility, affordability, and appeal of alcohol use disorder treatments and recovery models, and test strategies to increase their adoption in real-world settings
Although numerous effective alcohol interventions exist, there is a lack of data on their comparative effectiveness, how to optimize their dissemination and implementation among diverse groups and in multiple settings, and how to better integrate them with general medical care. There are concerted Federal efforts to facilitate health services research, and NIH has been at the forefront of supporting hypothesis-driven research on these topics and promoting effective, scalable implementation strategies to broaden health care access and use. NIAAA supports health services research designed to broaden the delivery of the full menu of evidence-based alcohol prevention and treatment services, such that individuals have greater access to interventions that address their specific alcohol-related issues in a wide array of care delivery settings. Innovations in collecting and aggregating clinical data collected within and outside health care settings, along with advances in research methods, have the potential to improve outcomes for individual patients, lower health care expenditures, and reduce the public health burden of alcohol misuse.
To identify and evaluate treatments and treatment delivery models that are appealing, affordable, accessible, and effective for different types of drinkers and for populations with special health care service needs, NIAAA will:
- Apply innovative clinical and health services research methods, such as adaptive research designs, pragmatic trials, and cluster randomized trials, to the development and evaluation of improved models for delivering alcohol-related care.
- Develop and evaluate strategies to reduce barriers that prevent people from seeking and receiving appropriate care for alcohol misuse and AUD and that enhance treatment adherence and retention.
- Develop and evaluate strategies to enhance the dissemination, implementation, and adoption of evidence-based treatment practices across the spectrum of services for at-risk and high-risk drinkers, as well as those with AUD and co-occurring conditions.
- Develop and evaluate AUD treatment and recovery models, including models tailored for nonmedical settings and medical nonspecialty settings, and models of care coordination between specialty treatment and general medical care settings.
- Develop and evaluate models for providing ongoing, comprehensive care for individuals with chronic AUD and other alcohol-related conditions within the health care system.
Highlights
NIAAA Wearable Alcohol Biosensor Challenge

In March 2015, NIAAA held a competition to create a better wearable alcohol biosensor device that could aid researchers, clinicians, therapists, and individuals by providing more accurate data on how much an individual is drinking. Current technologies for continuous alcohol monitoring, which are commonly used in the criminal justice system, are effective but cumbersome. Moreover, they only take readings every 30 minutes, and they reflect blood alcohol content (BAC) as it was 60–90 minutes prior to assessment—not in real time.
NIAAA’s challenge was designed to stimulate development of a sleek, unobtrusive sensor that could improve upon this interval and more closely approximate real-time BAC. The device was also expected to be able to interpret and securely store BAC data or transmit the data to a smartphone.
NIAAA announced the winner of the Alcohol Biosensor Challenge in May 2016. The winning prototype was submitted by BACtrack, a company known nationally for designing and selling portable breath alcohol testers for consumer and professional use. Their entry, the BACtrack Skyn, is worn on the wrist and, like existing continuous alcohol monitoring devices, detects alcohol through a person’s sweat. Significantly, it advances real-time alcohol monitoring by reflecting BAC as it was as little as 40 minutes prior to assessment.
Building on the success of the challenge, NIAAA issued a new challenge to stimulate inventors to develop a wearable biosensor that can detect alcohol directly in blood or the interstitial fluid that surrounds the body’s cells, rather than alcohol exuded in sweat. This method would measure a person’s current BAC.
Medications Development Continuum: A Bidirectional Process