A Protein-Based Diagnostic Seeds a New Business Venture
If you've ever needed a magnetic resonance imaging (MRI) scan, you may have had a contrast dye injected to create a clearer image. While MRI technology has improved greatly since its introduction in 1977, contrast dyes and the images they help produce have remained the same. InLighta BioSciences, a company supported through the National Institute on Alcohol Abuse and Alcoholism (NIAAA) Small Business Technology Transfer Research (STTR) program, is seeking to change that, starting with patients with liver disease.
After 25 years of researching calcium binding proteins and signaling pathways, InLighta BioSciences Founder, Principal Investigator, and Regents' Professor in the Department of Chemistry at Georgia State University, Jenny J. Yang, Ph.D., discovered a protein contrast agent (ProCAs®) that binds to calcium and provides improved contrast capabilities. She quickly realized this new agent had commercial potential, but she never planned to go into business herself.
"I thought a professor is a professor and I was happy as an educator and researcher," Yang says. "So becoming a businesswoman and having a startup company was not on my radar."
A Lab Tool May Be a Diagnostic Game Changer
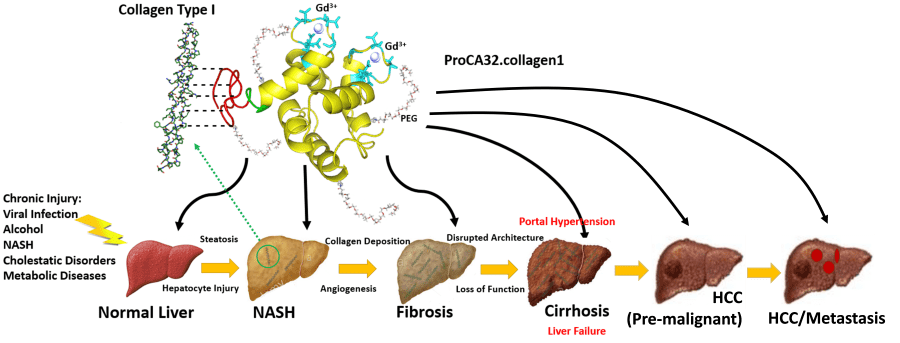
Normally, MRI contrast dyes are made of small, heavy magnetic metal and is not well-tolerated by all patients, especially those with kidney disease. The magnetism of the dye enhances the images taken by the MRI, revealing the differences between healthy and abnormal tissues through black and white images. Although practitioners can differentiate between normal and abnormal tissues, the dye will not discriminate between benign and diseased abnormal tissue and can only detect relatively large changes of about 1 cm. Dr. Yang realized that the protein she used to visualize calcium in the lab could work as a MRI contrast agent and it would be easier for patients to tolerate and possibly give better results.
Yang's contrast agent is a large molecule made from an altered human protein that senses and binds with calcium ions. Calcium is critical for maintaining bone health, muscle movement, and nerve impulses. Changes in the calcium can be an indicator of disease. The result is a clearer, more precise image that uses two colors to differentiate between benign abnormalities and those that are not. With much higher sensitivity and specificity, the protein-based contrast may lead to earlier detection of disease biomarkers such as calcium deposits, tumors, and fibrous or scarred tissue. It is also non-toxic, unlike heavy metal contrast agents.
"We can detect changes at stage 2 not stage 4 for fibrosis in liver disease," says Yang. "We double in accuracy."
InLighta Biosciences, which Dr. Yang founded in 2014 in Atlanta, Georgia, began by focusing on diagnosing liver disease, which causes severe fibrosis or scarring of the tissue. According to the Centers for Disease Control and Prevention, approximately 4.5 million U.S. adults have been diagnosed with liver disease. Heavy alcohol use is a risk factor for developing liver disease.
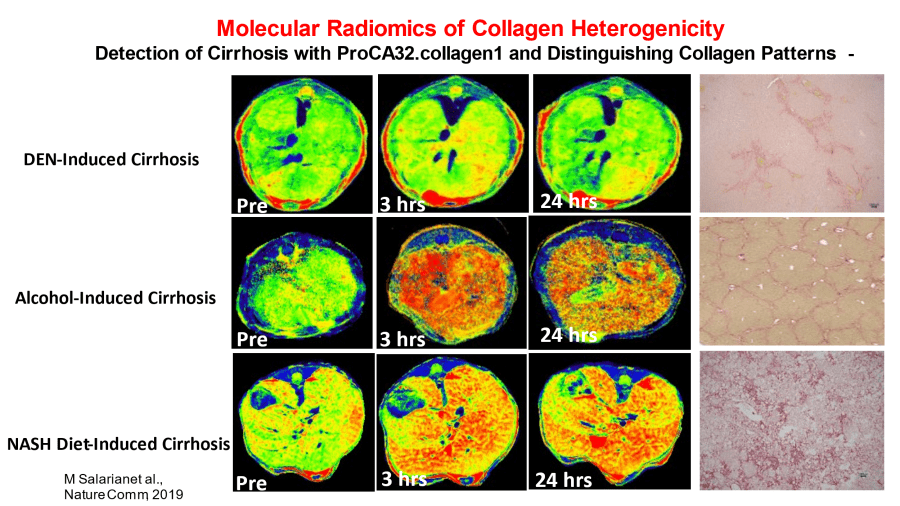
"We found that liver fibrosis has increased because we have obese and alcohol-related problems," Dr. Yang states. "The market is growing because now we are talking about a future problem." She adds that with the new technology, it is possible to not only visualize and diagnose fibrotic tissue, but to monitor and treat disease progression in patients over time.
An Education in Entrepreneurship
Convinced she had a "game-changer," Dr. Yang began looking for companies that would consider buying her technology. She says she faced two challenges: first there was little interest in marketing contrast agents, as opposed to more lucrative drug development and second, she had to convince people in the market that it would work.
"Like a lot of young innovators, Jenny was interacting with knowledgeable people but not necessarily the right people in the field," says Cliff Michaels, Ph.D., director of Georgia State’s Office of Technology Transfer.
After garnering little interest from pharma, she decided to start her own business and began searching for funding opportunities. With the help of Georgia State University’s Office of Technology Transfer, and after many drafts and revisions, she submitted a grant application to the STTR program at the National Institutes of Health. Eventually, NIAAA received her STTR application and provided InLighta the seed funding to start their journey.
"This funding allowed me to achieve a scientific breakthrough and now I can talk about early detection and I can talk about cancer, fibrosis and changing the industry," says Dr. Yang.
In addition to vital seed funding, Yang took advantage of every resource available, including local accelerators and training through Emory University and the Georgia Research Alliance, the Kaufman Foundation, an Emory University entrepreneurship program, and the NIH Commercialization Accelerator Program (CAP).
CAP helped her think clearly about how to advance to the next stages of commercialization, such as her target market and requirements of the U.S. Food and Drug Administration (FDA) to gain agency approval.
Dr. Yang confirmed she learned a lot from her Program Director as she moved through the STTR application and funding process. "You need to reach out for the help of a program director, find your resources, and be brave – don’t be afraid of rejection. They help you learn and prove yourself."

